How HAHN Automation Group helped one medical device manufacturer scale-up production while protecting IP
A large manufacturer of pathology solutions faced increased demands for their tissue-sampling probes; let’s call this customer, “Casey.” Casey forecasted volumes rising nearly 400% over the next five years—and they needed an additional end-of-line tester to meet those goals. Casey also wanted to add RFID tagging to protect their intellectual property as their products began to be sold internationally.
#automation #MedTech #RFID #pathology #surgicaldevices
- Improved cycle time with added features
- Intellectual property preserved
- Accuracy checked during production
Challenge
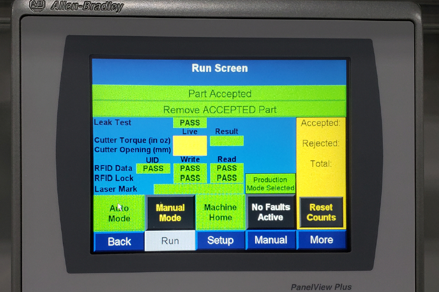
Casey needed a system that would meet the large and upcoming volume demands and improve testing consistency with an end-of-line tester. Additionally, Casey wanted to incorporate RFID tagging to protect intellectual property in their devices sold internationally.
Developing a Solution
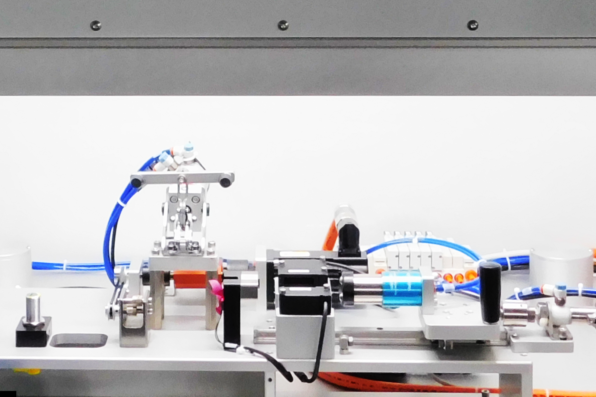
Having designed and built a previous test system for Casey, we looked for ways to enhance the original design to address the needs for improved cycle time and added security features.
We began by researching techniques for reading and writing to the RFID tags in a secure manner. Our controls engineers conducted feasibility checks and found that the sensor placement was critical in order to read/write without touching the handle of the device. This information was used to influence the design of the overall system.